Probiotics-Loaded Microspheres for Cosmetic Applications

The recommendation discloses an effective way of introducing live bacteria (a strain of L. casei) into cosmetic formulations where a method of encapsulation of the bacteria was used to increase their viability. Alginate microspheres were prepared for the systems to protect the microorganisms against external factors, such as temperature, UV light and preservatives. The obtained probiotic-loaded alginate microspheres were then used as the active ingredient of cosmetic formulations. Additionally, a preservative system was carefully selected to ensure the microorganisms’ viability and the microbiological stability of the products. The obtained results showed a significant improvement in the survival of the microencapsulated probiotic strain in the cosmetic formulations containing antimicrobial agents (6.13 log CFU/g after 120 days of storage) compared with the formulation containing the non-immobilised probiotic strain, where complete elimination of bacterial cells was observed.
Probiotics-Loaded Microspheres
The solution to the problem is provided by Cracow University of Technology & Lukasiewicz Research Network-Institute of Industrial Organic Chemistry
NPL Publication Year: 2024
Summary of the Solution
Technology Provider
- Cracow University of Technology
- Lukasiewicz Research Network
Technology used for microbiome stabilization: Alginate Microsphere Encapsulation
Microbiome Used in Cosmetic Composition/Product: Probiotic cultures (e.g., Lactobacillus casei)
Benefits of Stabilization Technologies
- Extended probiotic viability (6.13 log CFU/g after 120 days of storage)
- Protection from environmental stress
- Compatibility with cosmetic formulations
- Enhanced microbiome stability
Application Areas: Cosmetics, Pharmaceuticals, and Food Industry
Summary of method of encapsulation
- The aim of our current study was to provide evidence that the microencapsulation process can overcome the problem of the incompatibility of probiotic bacteria with cosmetic preservatives and can also enhance the viability of probiotic bacteria inserted into skin care products.
- Alginate microspheres were prepared for the systems to protect the microorganisms against external factors, such as temperature, UV light and preservatives. Alginate microspheres containing a Lactobacillus casei strain were obtained and then introduced into preserved cosmetic formulations.
- A preservative system was carefully selected to ensure the microorganisms’ viability and the microbiological stability of the products.
- The obtained results showed a significant improvement in the survival of the microencapsulated probiotic strain in the cosmetic formulations containing antimicrobial agents (6.13 log CFU/g after 120 days of storage) compared with the formulation containing the non-immobilised probiotic strain, where complete elimination of bacterial cells was observed.
Encapsulation of Lactobacillus casei for Stability
Why Encapsulation?
The use of the microencapsulation process can overcome the problem of the incompatibility of preservatives and extend the life of bacteria in cosmetic products.
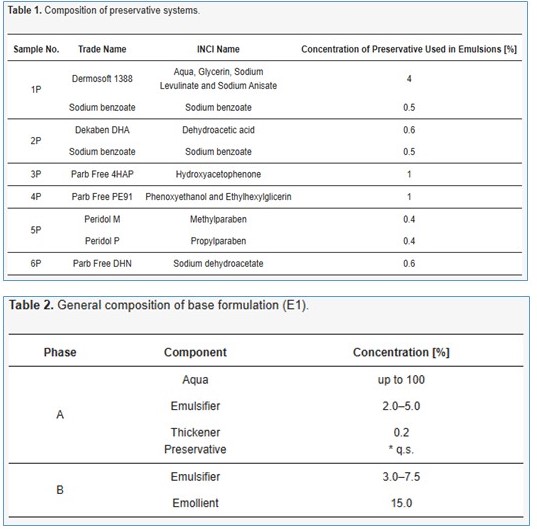
Other Composition used in cosmetic formulation:
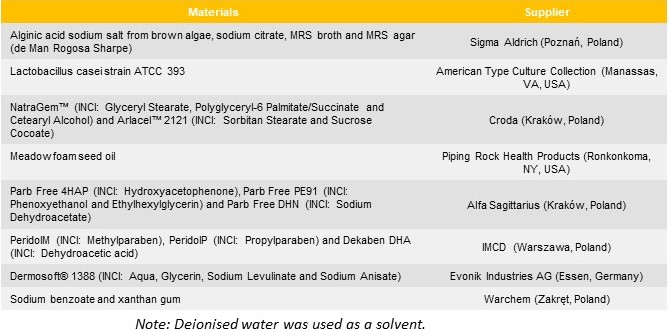
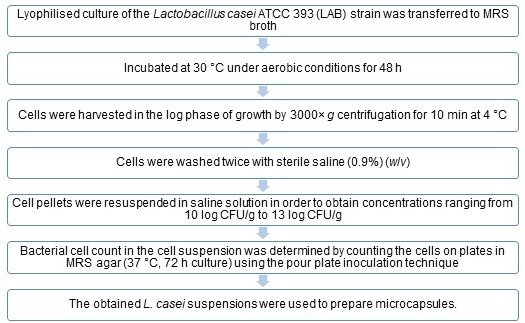
Materials and Methods
Encapsulation of Probiotics

Preparation of L. casei Suspension
Experiments and Results
Assessment of the Viability of Free and Encapsulated Probiotic Cells Depending on the Influence of Temperature UV light
Experiment Methodology
- Free and encapsulated probiotic cells were kept at 40 °C for 1 h and 96 h and at 50 °C for 10 min and were treated with UV light.
- The assessment of the impact of UV radiation on microcapsules was made according to the methodology proposed by Khorramvatan et al. with minor modifications.
- Samples (5 g) of each microencapsulated and free cell suspension (control) were placed on glass Petri dishes.
- The plates were then exposed to long-term UVB irradiation (311 nm) for 24 and 48 h. The UV sources were located 20 cm from each sample. Next, 1 g of encapsulated cells or 1 g of free cell suspension (initial density: 12.6 log CFU/g), stored under the conditions described above, was placed into 9 mL of 2 mol/L sterile sodium citrate (pH = 6) or 0.9% sodium chloride (w/v), respectively.
- After a series of dilutions, the viability of free and encapsulated cells was determined and compared.
- The same procedure was applied to microcapsules and free bacterial cells exposed to specific temperature conditions.
Result Estimation Method
- The experiments were repeated two times, and the results were used to calculate the average of log reduction.
- LAB cell reduction was calculated as the difference between the initial number of free LAB cells or LAB cells contained in the microspheres (No) and the number of free LAB cells or LAB cells contained in the microspheres exposed to physicochemical factors for a specific time (Nt).
Result
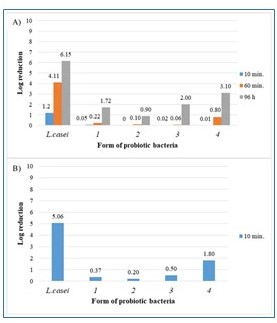
Figure 1. The effect of temperature on free and encapsulated Lactobacillus casei bacteria: (A) 40 °C and (B) 50 °C.
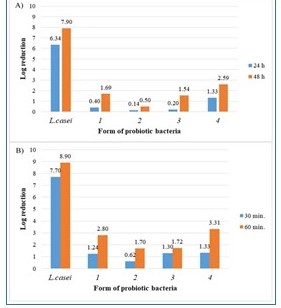
Figure 3. The effect of (A) UV radiation and (B) preservative on free and encapsulated Lactobacillus casei bacteria.
Assessment of the Viability of Free and Encapsulated Probiotic Cells Depending on the Influence of pH
Experiment Methodology
- In order to assess the survivability of bacteria in solutions with different pH values and the preservative solution, 1 g of encapsulated probiotics containing 9–11 log CFU of LAB cells was mixed with 9 mL of prepared solutions and incubated at room temperature (20 °C) for 15, 30 and 60 min.
- After incubation, the capsules were collected and put into 9 mL of 0.2 mol/L sterile sodium citrate (pH = 6).
Result Estimation Method
- Cell viability was assessed through pour plate inoculation and the counting of bacterial cells grown on plates with MRS agar at 37 °C for 72 h under aerobic conditions
Result
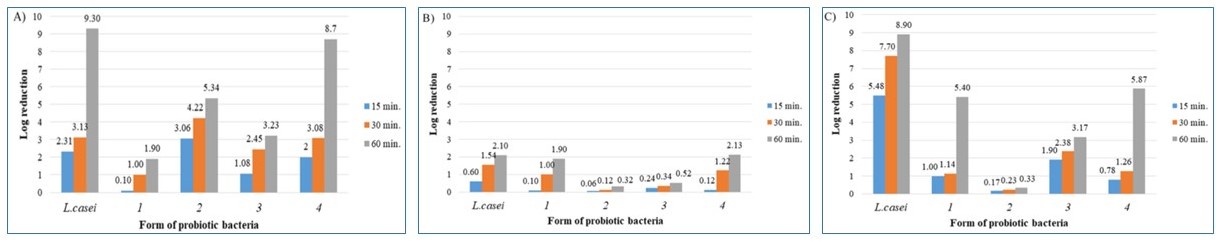
Figure 2. The effect of pH on free and encapsulated Lactobacillus casei bacteria: (A) pH = 2.0, (B) pH = 5.5 and (C) pH = 9.0.
Viability of Microencapsulated Lactobacillus casei in Skin Care Product
Experiment Methodology
- Microspheres that showed the greatest resistance to unfavourable physicochemical parameters were selected to use as the active ingredients in emulsion 3.
- However, the system that guarantees the highest survival of probiotic bacteria was selected as a preservative.
- The viability of probiotic cells in cosmetic formulations containing the free L. casei strain (E2) and L. casei microspheres (E3) was assessed immediately after introduction into the cosmetic and then after periods of seven days, one month and four months.
- The experiments were repeated two times, and the results were used to calculate the average log reduction.
- Cell reduction was defined as the difference between the initial amount of free LAB cells introduced into the cosmetic formulation (E2) or the amount of cells contained in microcapsules introduced into the cosmetic formulation (E3) and the amount of cells in the E2 formulation or E3 formulation after various storage times.
Result
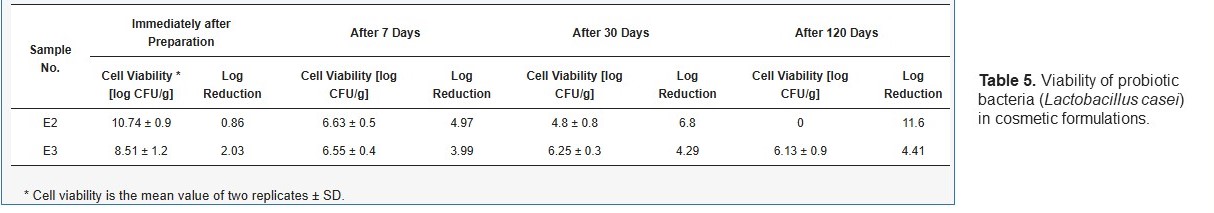
The obtained results enable the conclusion that encapsulation in the alginate microspheres ensures the survival of probiotic bacteria in the formulation, despite using the preservative system.
Advantages of Probiotic Encapsulation Technology
Key Benefits:
- The results showed that the encapsulation of bacteria in alginate microspheres could be an effective method of introducing live bacteria (strain of L. casei) into cosmetic formulations.
- Encapsulation enables the protection of microorganisms against external factors (temperature, UV light or preservatives) and, as a consequence, increases their viability.
- A significant improvement in the survival of the encapsulated probiotic strain in cosmetic formulations (6.13 log CFU/g after 120 days of storage) containing antimicrobial agents was observed in comparison with the formulations containing the non-encapsulated bacteria.
- Moreover, it has been shown that the selection of an appropriate preservative system is important in the case of cosmetics containing the probiotic strain of L. casei.
- For the developed products, systems based on organic acids and their derivatives proved to be the most effective antimicrobial agents because they had a weak impact on the degradation of microspheres; consequently, there was a decrease in the viability of the bacterial strain enclosed in alginate carriers.
- Taking into account the promising results, the elaborated technology may be an attractive approach to the development of long-stable cosmetic products containing live bacterial strains.
About Effectual Services
Effectual Services is an award-winning Intellectual Property (IP) management advisory & consulting firm offering IP intelligence to Fortune 500 companies, law firms, research institutes and universities, and venture capital firms/PE firms, globally. Through research & intelligence we help our clients in taking critical business decisions backed with credible data sources, which in turn helps them achieve their organisational goals, foster innovation and achieve milestones within timelines while optimising costs.
We are one of the largest IP & business intelligence providers, globally serving clients for over a decade now. Our multidisciplinary teams of subject matter experts have deep knowledge of best practices across industries, are adept with benchmarking quality standards and use a combination of human and machine intellect to deliver quality projects. Having a global footprint in over 5 countries helps us to bridge boundaries and work seamlessly across multiple time zones, thus living to the core of our philosophy - Innovation is global, so are we !!!
Solutions Driving Innovation & Intelligence
Enabling Fortune 500's, R&D Giants, Law firms, Universities, Research institutes & SME's Around The Globe Gather Intelligence That
Protects and Nurtures Innovation Through a Team of 250+ Techno Legal Professionals.