Hydrogen Storage Material Made from Magnesium Hydride

The present invention relates to materials used for the reversible storage of hydrogen. More particularly, the present invention relates to a compact composite material comprising a mixture of magnesium hydride and expanded natural graphite (GNE), in compacted form.
This material has reduced porosity, which increases its volume capacity for hydrogen storage. Its compact shape gives it mechanical strength which makes it easier to handle. The expanded natural graphite promotes the cohesion of the material which has a radial thermal conductivity much higher than that of magnesium or magnesium hydride powder. The composite can be handled in air without risk of spontaneous ignition even when it has been prepared with activated magnesium hydride.
The present recommendation is provided by “NAT CENTER FOR SCIENT RESEARCH (CNRS); GRENOBLE ALPES UNIVERSITY”
EP2244970B1, Granted & Alive Patent
Grant Date: May 24, 2017
|
Rank |
Recommendation focus |
Hydrogen Storage/ Transport Technology |
Process Parameters |
|
1 |
Method of making compacted material comprising magnesium hydride and expanded natural graphite; Method of H2 Storage & Release |
magnesium hydride and expanded natural graphite |
The pressure and temperature conditions can be easily determined from the equilibrium curve of magnesium hydride. |
|
Benefits |
|
Prior Art Limitations
- Metal hydrides are subject to the phenomenon of decrepitation after a certain number of hydrogen absorption/desorption cycles. Indeed, magnesium is ductile and therefore more easily accommodates the mechanical stresses induced by hydriding. Much research is currently being carried out to optimize the performance of magnesium hydride for hydrogen storage.
- The hydriding of magnesium is accompanied by a significant increase in volume of around 30%, which generates internal tensions and causes significant mechanical stresses on the walls of the tank.
- The powdered magnesium hydride has a low volume storage capacity and is difficult to handle.
- There is a need of significant work focused on improving the kinetics of absorption and desorption of hydrogen from MgH2.
Benefits over Prior Art
- The present reference discloses materials used for the reversible storage of hydrogen. More particularly, a compact composite material based on magnesium hydride for hydrogen storage and a process for its preparation.
- The aim of the present invention is to propose a material for the storage of hydrogen based on magnesium hydride which does not present the disadvantages mentioned, and in particular which is compact, can be handled without particular precautions, and has good properties in terms of mechanical strength, thermal conductivity and hydrogen absorption and desorption kinetics.
- The material used has reduced porosity, which increases its volume capacity for hydrogen storage. Its compact shape gives it mechanical strength which facilitates its handling. The composite can be handled in air without risk of spontaneous ignition even when it has been prepared with activated magnesium hydride.
Claim Elements
- The present recommendation by NAT CENTER FOR SCIENT RESEARCH (CNRS) & GRENOBLE ALPES UNIVERSITY discloses a Hydrogen Storage Material Made from Magnesium Hydride.
- A method for preparing a compacted material comprising magnesium hydride and expanded natural graphite, including the steps which consist of: activating the magnesium hydride or the powdered magnesium by co-grinding with a transition metal, a mixture of transition metals, a transition metal oxide or an alloy of transition metals, then
- mixing the activated magnesium hydride or activated powdered magnesium with a powdered expanded natural graphite;
- shaping the mixture by compaction in a pelleting machine.
- The magnesium hydride is activated with a centred cubic structure based on titanium, vanadium and a transition metal selected from chromium and manganese.
- The shaped material is then machined.
- The compacted material comprises from 80 to 99% by weight of magnesium hydride and 1 to 20% by weight of graphite.
- The recommendation also claims - A method for hydrogen storage and release from storage including the steps which consist in:
- introducing the compacted material into a suitable hydrogen tank;
- placing the material under hydrogen pressure in pressure and temperature conditions that enable the hydrogen to be absorbed by the material; and
- desorbing the hydrogen from the material in pressure and temperature conditions permitting the desorption of the material.
- In this method, the energy released by charging with hydrogen is evacuated by a heat exchanger.
Hydrogen Storage/ Transport Technology
- The present recommendation discloses the use of magnesium hydride and expanded natural graphite for Hydrogen storage.
- The magnesium hydride used for the manufacture of the material is in powder form, preferably having a particle size of between 1 and 10 μm.
- The powder mixture is compacted in a pelletizer.
- Activated magnesium hydride is advantageously in the form of a very fine powder, with a particle size of between 1 and 10 μm.
- This material has reduced porosity, which increases its volume capacity for hydrogen storage. Its compact shape gives it mechanical strength which facilitates its handling.
- Compaction makes it possible to increase the volumetric density of hydrogen storage compared to a fluidized bed of magnesium hydride and to improve the mechanical strength.
- Furthermore and surprisingly, the material thus obtained is no longer pyrophoric and can therefore be more easily handled.
- The shaped material can be machined, to present the dimensions adapted to the tank.
- The other properties of the material relating to the storage of hydrogen such as the kinetics of absorption and desorption of hydrogen are not significantly affected by the shaping of the material.
- The graphite is a natural expanded graphite (GNE). Expanded natural graphite is a form of graphite modified by chemical and thermal treatments. Graphite is advantageous because it is hydrophobic, refractory, a good conductor of heat.
- The expanded natural graphite promotes the cohesion of the material which has a radial thermal conductivity much higher than that of magnesium or magnesium hydride powder.
- The composite can be handled in air without risk of spontaneous ignition even when it has been prepared with activated magnesium hydride.
Method/Process Details
- First Aspect: A first aspect, the invention proposes a compacted material obtained by the process according to the invention and comprising magnesium hydride and expanded natural graphite.
- Magnesium hydride can in particular be obtained by an incomplete hydriding reaction. Advantageously, the magnesium hydride powder used contains less than 10% by weight, preferably less than 5% by weight, of metallic magnesium. In fact, the powder will be all the more stable with respect to air if the magnesium hydride is perfectly hydrided
- The magnesium hydride is activated beforehand in order to present favorable hydrogen absorption and desorption kinetics. This activation is carried out by co-grinding the magnesium hydride with a transition metal, a transition metal alloy or a transition metal oxide preferably introduced in proportions of between 1 and 10 atomic% relative to the mixture, preferably around 5%.
- The co-grinding of the magnesium hydride with the activation agent is carried out in the absence of expanded natural graphite. In fact, crushing expanded natural graphite would have the effect of destroying the “skeleton” and the layers of the GNE. However, it is preferable that the structure of the GNE in the form of sheets is preserved to give the final compacted material a strongly anisotropic character.
- Furthermore, the compacted material according to the invention is homogeneous whatever the proportion of GNE. Homogeneity results from a small difference between the density of the hydride and that of the GNE. Indeed, GNE has a low density and the magnesium hydride activated by an activating agent (transition metal, a transition metal alloy or a transition metal oxide) is very powdery following co-grinding and therefore has an apparent density closer to that of GNE than that of metal hydrides. As an indication, the density of activated magnesium hydride is approximately thirty times lower than that of LaNi5Hx. Thus, the preparation of compact LaNi5Hx/GNE materials is much more delicate and their homogeneity is more uncertain.
- Second Aspect: Also, according to a second aspect, the invention proposes a process for preparing a compacted material comprising magnesium hydride and expanded natural graphite, comprising the steps consisting of:
- mixing a magnesium hydride or powdered magnesium with expanded natural graphite; and
- shaping the mixture by compaction in a pelletizer.
- The above preparation process first comprises an additional step consisting of activating the magnesium hydride or powdered magnesium by co-grinding with an activation agent chosen from a transition metal, a mixture of metals of transition, a transition metal oxide and a transition metal alloy. This process thus makes it possible to prepare a compacted material comprising activated magnesium hydride and expanded natural graphite. The magnesium hydride used for the manufacture of the material is in powder form, preferably having a particle size of between 1 and 10 μm. The powders can be mixed in a conventional manner, for example in a mixer. It is preferably carried out at room temperature and at atmospheric pressure.
- The powder mixture is compacted in a pelletizer. Advantageously, the mixing and compaction are carried out under a controlled atmosphere, in particular when pyrophoric activated magnesium is used.
- The force exerted during compaction is chosen in particular as a function of the desired porosity in the material. As an indication, a compression force of the order of 1 t/cm<2> proved to be appropriate for obtaining pellets of material having a porosity of the order of 0.3.
- Compaction makes it possible to increase the volumetric density of hydrogen storage compared to a fluidized bed of magnesium hydride and to improve the mechanical strength. Furthermore and surprisingly, the material thus obtained is no longer pyrophoric and can therefore be more easily handled.
- The shaped material can then be machined, in particular to present the dimensions adapted to the tank. The other properties of the material relating to the storage of hydrogen such as the kinetics of absorption and desorption of hydrogen are not significantly affected by the shaping of the material. The material described is easily handled, even when it incorporates an activated magnesium hydride and has an improved hydrogen storage volume capacity.
Method/Process Details
- Final Aspect: According to a final aspect, the invention finally proposes a process for storing and releasing hydrogen comprising the steps consisting of:
- introducing a described material into a suitable hydrogen tank;
- pressurizing the material with hydrogen under pressure and temperature conditions permitting the absorption of hydrogen into the material; and
- desorbing hydrogen from the material under pressure and temperature conditions corresponding to the desorption of the material.
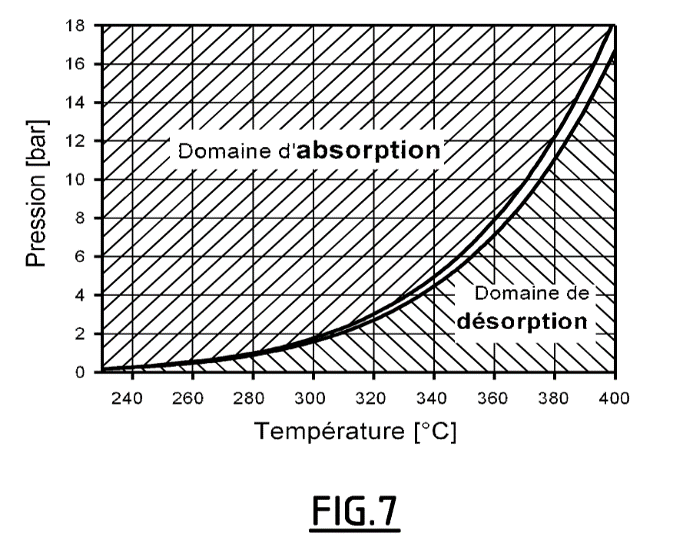
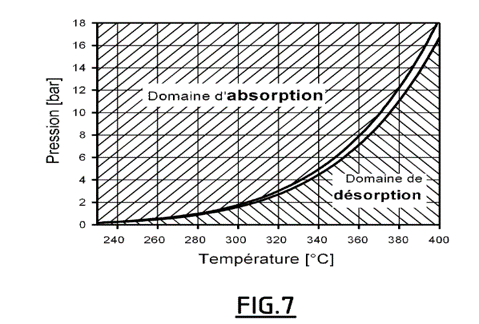
- Advantageously, the energy released by the hydrogen loading is evacuated by a heat exchanger. The pressure and temperature conditions can be easily determined from the equilibrium curve of magnesium hydride (see Figure 7).
Process Parameters
- The powders can be mixed in a conventional manner, for example in a mixer. It is preferably carried out at room temperature and at atmospheric pressure.
- The mixing and compaction are carried out under a controlled atmosphere, in particular when pyrophoric activated magnesium is used.
- The force exerted during compaction is chosen in particular as a function of the desired porosity in the material. As an indication, a compression force of the order of 1 t/cm<2> proved to be appropriate for obtaining pellets of material having a porosity of the order of 0.3.
Proof of Concept
Example – 1: Preparation of MaH2/GNE pellets
In a suitable sealed mixer, placed in a glove box under a controlled atmosphere, 47.5 g of activated magnesium hydride powder with an average particle size of 1 to 10 μm are carefully mixed. , (available from MCP MG Serbien or MCPHy Energy SA) with 2.5 g of expanded natural graphite powder (GNE, medium particle size in the form of particles with a length of a few millimeters (available from SGL Technologies GmbH ).
The mixture of powders (50 g) is then poured into the matrix of a hardened steel pelletizer also placed in the glove box. The pelletizer is taken out of the glove box in an airtight bag and placed under it.
The powder placed in the pelletizer is compacted by uniaxial compression with an intensity of the order of 1t/cm<2>(10<8>Pa), gray, compact material, with an appearance close to that of solid graphite with a diameter of 8 cm, which can be handled in the open air for a few minutes. Nevertheless,It is preferable to store composites in a controlled atmosphere to avoid any risk of heating and oxidation on the surface.
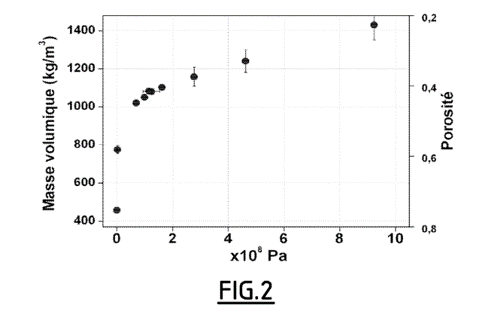
The density of the material in the different pellets obtained was calculated by weighing and measuring the dimensions. The porosity was calculated from the theoretical and measured densities. The results are shown in Figure 1.
The pellets have sufficient mechanical strength and oxidation stability to allow their handling for the purposes of introduction into the hydrogen tank under normal conditions, that is to say in particular outside the glove box.
The pellets obtained have exceptional mechanical strength. It is thus possible to machine them, for example to adjust the external diameter to the diameter of the tank, or to drill holes to insert heating elements and thermocouples. Likewise, after repeated hydrogen cycling, neither cracking nor accumulation of fine powders at the bottom of the tank is observed.
The presence of GNE promotes the cohesion of the compressed material (figure 2). Indeed, pellets prepared without GNE are more fragile and do not allow subsequent machining.
A photograph of a pellet obtained
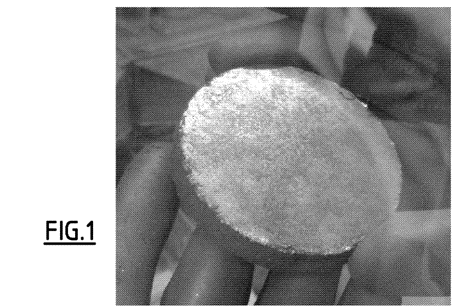
Fig.2: The measured density and the corresponding porosity of pellets according to example 1 as a function of the compressive force applied;
Example – 2: MaH2/GNE application tests and comparison with MgH2
The properties in the hydrogen storage application of the material obtained in Example 1 were evaluated and compared to those of magnesium hydride activated powder.
- The diameter of the MgH2/GNE pellets obtained in Example 1 was reduced from 8 cm to 7 cm by machining. 250g of these pellets were introduced into a cylindrical stainless steel tank with an internal diameter of 7 cm and a volume of 270 cm<3>. The tank is equipped with heating means. The tank was then heated to a temperature of 300°C and pressurized with 8 bars of hydrogen.
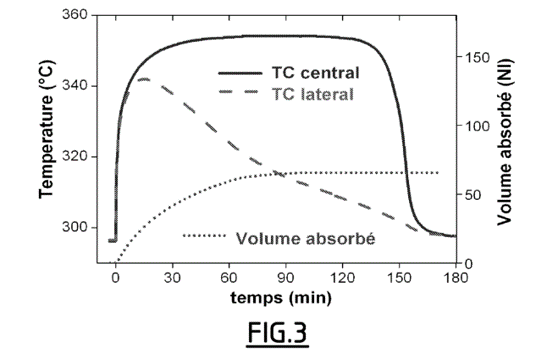
- The volume of hydrogen absorbed was recorded by flow meter for 3 hours. The temperature was monitored using probes placed in the center and periphery of the tank.
- The test is carried out under the same experimental conditions, except replacing the pellets according to Example 1 with 110 g of activated magnesium hydride powder.
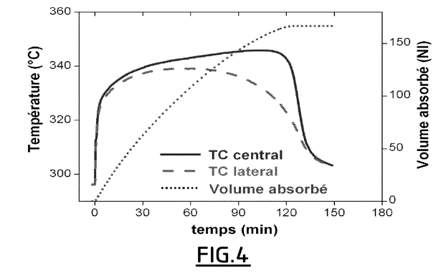
- Figures 3 and 4 show that the volume of hydrogen absorbed goes from 65 NL with the powder to 170 NL with the pellets (Normal Liters). Compressing the powders therefore makes it possible to more than double the hydrogen storage volume capacity of the material. Comparison with Figure 4 further indicates that loading is faster for compacted material, while the mass of the material and therefore the quantity of heat to be evacuated is multiplied by a factor of approximately 2.5.
Comparison of the temperatures recorded at the center and at the periphery of the reservoir for the pellets (see Figure 4) and the powder (see Figure 3) also shows that the temperature is much more homogeneous within the material according to the invention.
The volume of hydrogen absorbed when loading the tank filled with MgH2 powder and the temperature measured in the center and periphery of the tank;
The volume of hydrogen absorbed during loading of the tank filled with pellets obtained according to example 1 and the temperature measured in the center and periphery of the tank
Results:
- These results demonstrate that the material described makes it possible to significantly improve the radial thermal conductivity and the volume storage capacity of the hydrogen storage material.
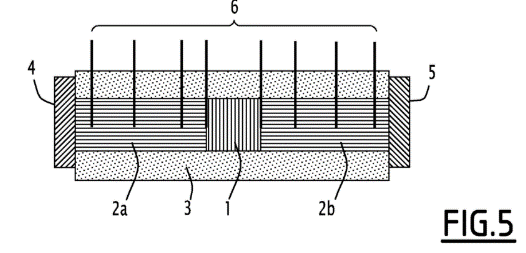
- The thermal conductivity was measured for samples prepared with different GNE contents. The measurements were carried out on a conventional steady-state measuring bench based on the split-bar principle (see Figure 5). The sample is positioned between two standard pieces 2a, 2b and surrounded by insulation 3, the whole being in contact on either side with a hot plate 4 and a cold plate 5. Thermocouples 6 are inserted into the standards and the sample in order to record the temperature depending on the distance between the plates.
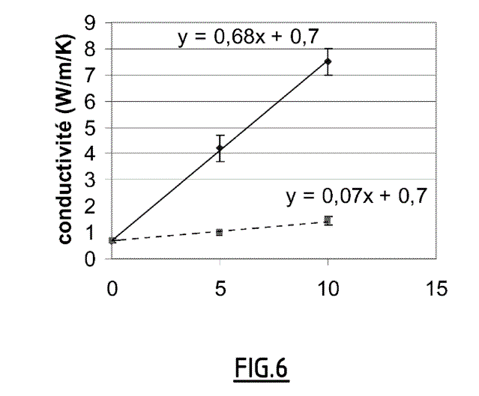
- The average temperature of the sample during the measurements is around 30°C. Three compositions comprising 0%, 5% and 10% (by weight of GNE respectively) were tested. To this end, “bars” were cut in the plane of the pellet (radial measurements reported in a continuous line in Figure 6), and along the axis of compaction (axial measurements, in a broken line).
- The results are illustrated in Figure 6 and indicate that for the contents studied, the thermal conductivity is proportional to the GNE content. We also observe a very strong anisotropy of behavior: the radial conductivity increases very quickly with the GNE content, while the axial conductivity is only very little affected by the presence of GNE.
- Furthermore, the material described has low reactivity to air, which makes its handling much safer and in particular facilitates the loading of the hydride tanks.
- Its very good mechanical strength also allows the machining of parts to adapt them to the geometry of the tanks, for example in order to provide for the passage of heat exchangers. The mechanical strength persists even when cycling under hydrogen, which makes it possible to avoid mechanical stress problems associated with powder compaction and/or decrepitation.
Diagram of thermal conductivity measurement according to the divided bar principle
The radial and axial thermal conductivities of the material obtained according to Example 1 for different graphite contents
The Mg-MgH2 equilibrium curve allowing the absorption and desorption conditions to be determined
NAT CENTER FOR SCIENT RESEARCH (CNRS)– Commercial Technology
Commercial Technology
Objectives: Large capacity storage facilities in terms of weight and volume, combining high energy efficiency, safety and competitive price.
Main research areas:
- Reversible solid storage at room temperature in metal and complex hydrides.
- Solid storage in porous and hybrid materials with high specific surface area.
- Regenerable hydrides with high weight capacity, hydrogen generation by thermolysis and hydrolysis, regeneration processes.
- Liquid hydrogen storage: cryogenic hydrogen and new hydrogen carriers NH3 and LOHC.
- Conformability and thermomechanical resistance of high-pressure tanks.
- Modelling of materials and storage tanks.
- Catalysts for hydrogen generation and incorporation.
- Decrease of critical raw material content, recycling processes.
- Optimisation of energy efficiency in the direct coupling of the electrolyser and the fuel cell.
Hydrogen Storage on Solid Disks
- A group of French researchers has made a significant breakthrough in hydrogen storage technology. Unlike traditional methods of storing hydrogen in gas or liquid form, they have been able to store hydrogen on solid disks.
- The solid disk storage method developed by a group of French researchers (Albin Chaise, Patricia de Rango, Daniel Fruchart, Michel Jehan et Nataliya Skryabina) offers a more efficient and secure alternative.
- The initial research was conducted at the French National Center for Scientific Research (CNRS), which filed the first European patents. The technology was then transferred to two industry partners, McPhy and JOMI-LEMAN, in order to start the commercialization of the product. This innovative solution could facilitate hydrogen transportation, addressing key challenges in the field.
- To store and release hydrogen, the disks only require heating to a temperature of 300 degrees Celsius. Additionally, the loading and unloading of hydrogen occur at very low pressures, 10 bars for charging and 2 to 3 bars for discharging. This will reduce the risk of accidents during storage and transportation, making it a promising solution for widespread adoption.
Magnesium Hydride
The CNRS said that the French researchers have utilized magnesium hydride (MgH2) as a key material for storing hydrogen in the disks. The addition of 5% of expanded graphite to the mixture helps manage heat release during hydrogen absorption and desorption.
The disks store both hydrogen as an energy carrier and the heat generated during the reaction, maintaining it for six to eight hours for subsequent use during discharge.
The researchers have demonstrated an energy efficiency of 80% with a tested lifespan of 7,400 charging and discharging cycles, projected to exceed expectations.
Advantages of Solid Disks
- Indeed, this solid storage method surpasses the safety standards of gas or liquid storage techniques. It can indeed operate at low pressure, eliminating the risk of reactions with the surrounding air. Besides, it requires less energy compared to conventional storage solutions.
- One of the notable advantages of the solid disk storage method is its enhanced safety features. Traditional methods of hydrogen storage, such as high-pressure gas tanks, can pose risks of leakage or combustion. The solid disks, however, can be safely handled at ambient temperatures without any reaction with the surrounding air.
- This revolutionary approach offers enhanced safety and efficiency, potentially transforming the entire industry.
- The solid disk storage method offers a more efficient and secure alternative.
Applications and Potential
- The applications for this innovative hydrogen storage technology are wide-ranging. In comparison to compressed gas or liquid storage, the solid-state disks require less energy and enable higher storage density.
- The storage density of the disks reaches almost two and a half times the density of conventional highly compressed hydrogen storage (106 kg of hydrogen per cubic meter compared to 42 kg per cubic meter at 700 bars). Therefore, this solution is specifically designed for mass hydrogen storage, opening up vast possibilities for a range of applications including refueling stations, industrial processes, and even the production of fertilizers.
- Already, the disks have been successfully commercialized in Italy and Japan, and discussions are underway in Norway to adapt the system for use in ferries, mass-scale maritime transportation, and large chemical industries.
About Effectual Services
Effectual Services is an award-winning Intellectual Property (IP) management advisory & consulting firm offering IP intelligence to Fortune 500 companies, law firms, research institutes and universities, and venture capital firms/PE firms, globally. Through research & intelligence we help our clients in taking critical business decisions backed with credible data sources, which in turn helps them achieve their organisational goals, foster innovation and achieve milestones within timelines while optimising costs.
We are one of the largest IP & business intelligence providers, globally serving clients for over a decade now. Our multidisciplinary teams of subject matter experts have deep knowledge of best practices across industries, are adept with benchmarking quality standards and use a combination of human and machine intellect to deliver quality projects. Having a global footprint in over 5 countries helps us to bridge boundaries and work seamlessly across multiple time zones, thus living to the core of our philosophy - Innovation is global, so are we !!!
Related Resouce: Hydrogen Storage/Transport Technology: Overview and Market Insights
Solutions Driving Innovation & Intelligence
Enabling Fortune 500's, R&D Giants, Law firms, Universities, Research institutes & SME's Around The Globe Gather Intelligence That
Protects and Nurtures Innovation Through a Team of 250+ Techno Legal Professionals.